The rusting process cannot occur without the movement of ions; for metal to rust, it has to be able to give off ions. The slower it can give off ions, the slower it can rust, and the faster it can give off ions, the faster it can rust. When a metal is dipped or soaked in saltwater, its ions begin to move faster, and the metal gives off the ions faster. Due to this, rusting occurs. So, how does salt water affect rusting?
Saltwater affects rusting by speeding up the process. Saltwater is an electrolyte that contains more dissolved ions than distilled water. As a result, it gives off more dissolved ions and enables electrons to move faster.
Furthermore, moisture and oxygen are a trigger for rust. Exposure to water will enable the moisture present on the metal to react with the oxygen present in the atmosphere. This process results in oxidation and leads to rust, a common name for iron oxide.
Contents
Can A Metal Rust Without The Presence Of Oxygen?
No, a metal will not rust without the presence of oxygen. If iron or any metal containing iron is exposed to any environment that is rich in oxygen and catalyst friendly, then the process of oxidation begins.
Air is not always important for rust to occur, only oxygen is and oxygen can be found in water also. Water is in fact important for the oxidation process because it is what allows the iron to meet with the oxygen. This is the reason more moisturized areas have rusty metals than dry areas.

Rust is majorly oxidation, or a chemical reaction that involves oxygen. When oxidation takes place in some elements, a very thin film is formed as a result, for example the green layers you can find on copper. Other elements such as iron show rust as its own proof of oxidation.
The iron molecules on the surface of the metal object will exchange it’s atoms with the oxygen present in air and the atoms left will form a new substance often reddish or brownish, which we know as rust.
How Does Rust Work?
Rust is the general name for iron oxide, a very common compound. This compound is common because iron combines readily with oxygen so easily that you can hardly find pure iron in nature. Iron or steel rusting is a very common example of corrosion.
Only metals can corrode. When a piece of metal corrodes, an electrolyte helps to provide oxygen. As oxygen combines with the metal, electrons are let loose and they flow through the electrolyte becoming converted into what is known as rust as they do.

For iron to achieve this phase, however, three things are needed; iron, water or moisture and oxygen.
When moisture touches an iron object, the moisture first serves as a good electrolyte, combined with the carbon dioxide present in the air to form an even stronger electrolyte. As the acid forms and the iron dissolves, some of the water (moisture) breaks down to form hydrogen and oxygen.
The free oxygen and dissolved iron bond into iron oxide, forming free electrons in the process. The electrons liberated from the anode portion of the iron then flows into the cathode, which may be another point on the iron itself.
Does Metal Rust In The Deep Sea?
For iron to rust, both water and oxygen must be present. Scientifically, iron loses a few electrons to oxygen atoms. This is called the oxidation process. When oxidation occurs, rust is produced.
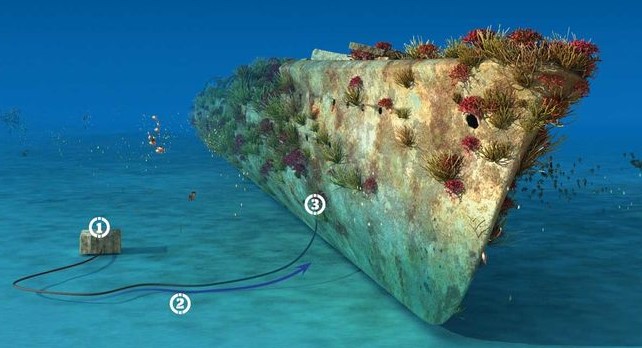
Steel rusts faster in saline water than in ordinary water. The presence of salt acts as a catalyst, accelerating the corrosion process. A metal might however not rust in the deep sea because of the accretions of calcium carbonate that form protective coatings on the metal. If these metals are however exposed to air, they would break down within a short period.
Can You Prevent Rust By Applying Oil?
Yes, lubricants prevent rust and oil is a form of lubrication. Oil can be used to protect and shield the lifespan of machinery surfaces by preventing oxidation and reducing the occurrence of rust among other things.
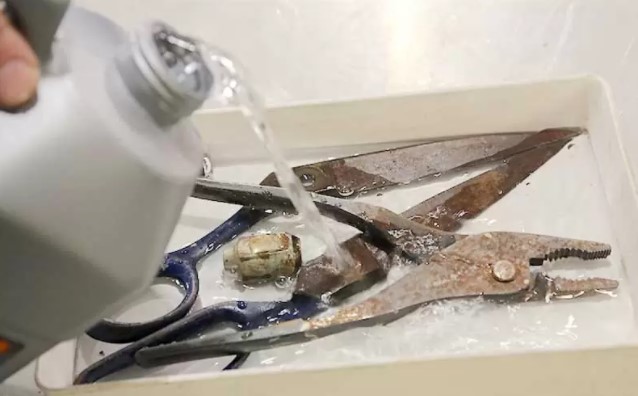
However, not all types of oil can be applied to any metal surface and using the wrong type of oil for lubrication can damage your metal, hence it is important to know the right type of oil or instead seek professional advice.
Staving off and preventing corrosion and rust by proper lubrication is a critical part of successfully maintaining any metal. It will help maintain the health of your machinery and save costs.
Does Paint Prevent Rust?
Yes, paint prevents rust. Rust is weak, and as a result of this, paint will prevent it.
However, it is important to remove all resisting rust from the metal surface before attempting to paint it. This is because if the paint film over the rusty part breaks, water can come in contact with the metal and start to corrode it, resulting in destruction of more of the paint film. It is therefore necessary to scrape out all the rust from the surface of the metal before applying paint.
Will Salt Water Corrode Aluminum?
Yes, saltwater will corrode aluminum. When salt air or salt water comes into contact with aluminum they can cause the aluminum to give off white, chalky coating of aluminum oxide and unpleasant pitting.
However this can be avoided by using a specialized form of powdered coating or galvanizing.


