Electrolysis is the process by which ions are transferred from one solution to another using electricity. Electrolysis is widely used in various industries worldwide, from metal plating and stripping to petroleum refining and water treatment. However, how does electrolysis work to remove rust?
Electrolysis works by running a current through a water bath with an electrical charge added to it. The electrical charge helps the electrolyte solution conduct electricity and breaks the rust down into its oxides. The result is a clean metal that can be primed, painted, or sanded to remove any remaining residue.
Electrolysis removes rust by transferring electrons from the metal atoms in rust to a sacrificial anode made of another metal. The process can make large objects like boat propellers look good as new.
You can read further for more information on how electrolysis works on metals to remove rust.
Contents
What Is Electrolysis?
In simple terms, electrolysis is a technique through which ionic substances are broken down into smaller particles when an electric current is made to pass through them. When an electric current passes through a substance, a chemical change occurs. The chemical change could either be a loss or a gain in electrons.

Electrolysis is widely used in the metallurgical industry to extract and purify metals from metal ores. It is used in electroplating.
Benefits of Using Electrolysis to Remove Rust
Other rust removal methods like abrasives and sandblasting clean metals indeed, but they are not very efficient for old or valuable artifacts because of their side effect on the metal. These methods are sometimes destructive, and they tend to remove the excellent metal and the rust. Rust can be removed using strong acids, but these also affect the metals negatively.

- It is a quick and easy way to remove rust from any ferrous object
- The electrolytic rust removal method is a cheap and safer method of removing both light and heavy rust
- It is a time-saving process
Electrolytic rust removal is the best option to remove rust from your metals without losing the metal.
How Electrolysis Works to Remove Rust?
Rust removal using electrolysis is possible with current flowing through a fluid electrolyte to generate charged (positive and negative) ions on two metals.
A substance (the rusted metal) consisting of negative and positive electrodes is held and dipped into a liquid containing negative and positively charged ions. The portion with the negative charge absorbs the ions from the positively charged part. The outcome of this process is that the negatively charged portion (also known as the anode) decays, while the positive charge portion (also known as the cathode) is treated of all rust
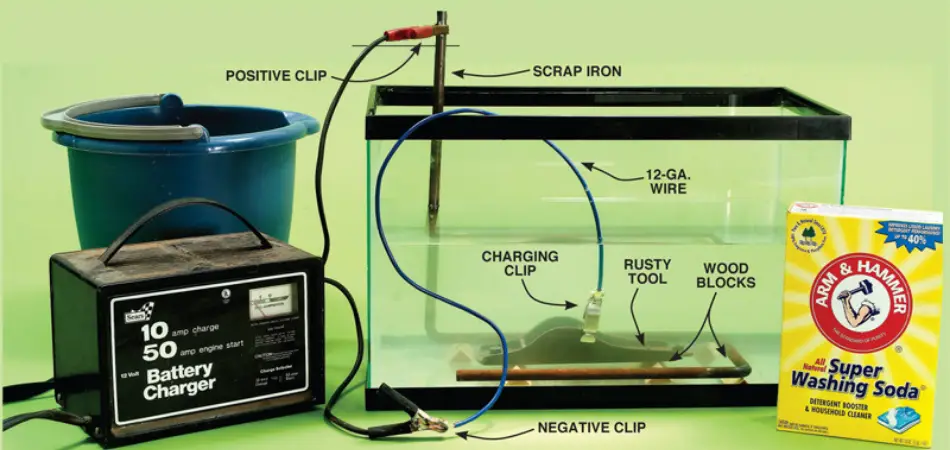
Materials Needed for Electrolysis
Before you begin the process, ensure you are in an adequately ventilated area. This is because electrolysis emits some amount of hydrogen and oxygen gases that are flammable.

To begin the process of rust removal using electrolysis, you will need the following materials.:
- A piece of scrap steel
- A plastic container: a plastic container is preferred because it is nonreactive. Get a large plastic bucket that will be used to prevent the tool and the anode from coming in contact
- A battery or a battery charger: a minimum of 12 – volt battery charger
- And the metal to be cleaned.
- Some electrolyte: a washing soda (1/3 cup washing soda) is the best electrolyte to add to your liquid for dust removal
- Water solution: warm water that will mix and dissolve the electrolyte. Tap water is okay for the process.
Step 1: Begin by disassembling the rusty item as much as possible. Also, keep wood and leather parts away from the water
Step 2: mix the electrolyte solution (washing soda) until it is completely dissolved
Step 3: suspend the rusty metal in the electrolyte solution
Step 4: position the anode in the plastic container so that one end sticks out of the liquid. Place it a couple of inches away from the rusty item and ensure they do not make contact.
Step 5: Connect the negative clamp (identified with a black colour) from the battery charger to the rusty metal. If in the process, the rusty metal sticks out of the water.
Step 6: Now connect the positive clamp (red colour) from the battery to the portion of the negative anode that sticks from the water. Try not to mix the two clamps.
Step 7: Turn on the battery charger. Avoid touching the battery while it is running.
Step 8: As the battery runs, the metal and the anode will soon emit tiny bubbles. The little bubbles you see on the metal helps to loosen and flake off the dust. And gradually, the rust begins to fade out or disintegrate. Depending on the size of the rusted item, you can leave your item for up to 5 hours, and some more oversized items can stay up to two days.
Step 9: when you are convinced you have given enough time to the process, you can turn off the battery charger and remove the steel item from the electrolyte solution. When you remove the item from the solution, you will discover a powdery black iron. You can use a ball of steel wool, a scrubby sponge, or a brush to wipe the black coating. You can repeat the process if you find additional rust after wiping the item.
Conclusion
Electrolysis is an effective way to get rid of rust once you have mastered how to go about it, and you will be amazed at how perfectly it works as long as there is rust. Although some people have argued that there are drawbacks to electrolysis, especially if the metal is left in the electrolyte solution for long, it may become brittle. However, this can be curbed by allowing the treated material to rest for a good number of hours before use.

