Bronze is a copper-based metal alloy that has been used for thousands of years, and alloys with small amounts of tin, lead, zinc, or silicon are commonly found in home decor products. There are several reports that bronze does rust, but of the truth, does bronze rust?
No, bronze does not rust. Rust is iron oxide, formed when iron reacts with moisture and oxygen (present in the air or atmosphere) from its environment. Bronze contains no iron; it is instead an alloy of tin and copper. It might include a few other metals, such as zinc, but these are usually minor additions, and the major constituents hardly ever vary. As a result of this, bronze cannot rust.
It is often said that bronze does not rust. However, bronze is a copper alloy, and copper does tarnish. With time and exposure to the elements, all copper alloys (bronze) will tarnish.
In some environments where regular maintenance is difficult, it might not be the right material choice for an outdoor sculpture, but luckily bronze sculptures can be easily cleaned and maintained to keep them looking bright and new.
If you are still skeptical on if bronze rust or not, I suggest you keep reading, and this post covers all that you need to know about bronze and how it reacts to rust reagents.
Contents
What Is Rust?
Rust is a form of corrosion of iron. This occurs when iron comes in contact with moisture and this moisture reacts with oxygen leaving a reddish surface.

Rust is simply the common name for the oxide of iron. When Iron is exposed to water or moisture without any treatment, the moisture present on the surface of the iron reacts with oxygen which is present in the air or atmosphere. The reaction of the water on the surface of the metal and oxygen spurs oxidation which results in flaking and a reddish or blackish red substance on the iron, what we commonly refer to as rust.
Why Doesn’t Bronze Rust?
Bronze does not rust as a result of its lack of or minimal iron content. However, bronze can react with oxygen in other ways. Bronze is not a naturally occurring element like iron or pure aluminum. Instead, it is majorly a mixture of copper and tin. This structure of bronze makes it more resistant to rusting or corrosion although this does not mean it is completely immune. Bronze can still deteriorate as time passes even though it might take a long while.
Bronze is often referred to as an alloy of about 12% tin and 88% copper. Although bronze may contain manganese, phosphorus, arsenic, aluminum foil, silicon, nickel, or zinc, it does not contain iron and so it cannot rust.
Does Patina Form On Bronze Objects?
Yes, patina forms on bronze objects. Shortly after a bronze object has been produced, a very thin layer of patina forms on the surface of the object. This patina is usually brown and protective. Depending on the accessibility of moisture and other agents, this patina may subsequently and slowly become evenly green or blue. This process can sometimes take as long as fifty years.
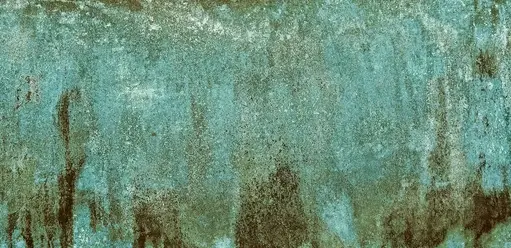
Modern environments are much less friendly to outdoor bronzes. This is as a result of pollutants, sometimes the products of solid and liquid fuel burning, in conjunction with moisture, can result in rapid corrosion.
This process is usually very fast and harsh and produces visually awful results; A mottled, pitted, streaked, or powdery green surface. Pollution also causes deposits of first, which further enhances corrosion on the bronze’s surface.
Which Other Metals Are Rust Proof?
Here is a list of other metals that are rust proof.
Galvanized Steel
Galvanizing is a detailed process used to protect iron or steel from undergoing rust by coating it with a layer of zinc. This is mostly achieved by dipping the steel in a molten zinc bath that has been heated to a temperature of over 450°C. Other methods of galvanization are however still good for steel, such as electroplating.

Galvanizing steel is however not hundred percent perfect as the protective layer can be damaged over time. However, galvanization is still a little more workable and cheaper process than stainless steel and offers better rust protection over time than weathering steel.
Aluminum
Aluminum is an extremely abundant and versatile metal and does not rust because it does not contain iron, except it is alloyed with iron. Aluminum reacts with oxygen in moisture or water, but the aluminum oxide acts as a thin corrosion-resistant defensive layer, protecting the metal from further damage.
Brass
Brass is an alloy of copper and zinc, neither of which can rust. It is also stronger than pure copper. The increased strength of brass combined with good corrosion resistance makes it a good choice for major applications. Brass is also a smooth metal, malleable, and aesthetically pleasing. As a result, it is a major choice for decorative items, home fittings, and musical instruments.

Copper
Copper is a naturally occurring metal, meaning it can be found in nature in its pure metallic form. Copper does not rust, but it does oxidize slowly when exposed to air, ultimately resulting in a green layer known as patina. As with aluminum, this layer preserves the metal but is commonly not regarded as an attractive look.
Stainless Steel
Stainless steel that contains high enough chromium levels do not rust because the chromium will oxidize faster than iron, creating a layer of chromium oxide and preventing the formation of rust. Adding nickel further enhances the rust proof qualities in the alloy.
Is Bronze Prone To Corrosion?
No, Bronze is not prone to corrosion. Bronze is a mixture of copper and tin, along with minor amounts of other elements, and is naturally much more resistant to corrosion than copper. Bronze can tarnish, but it does not rust, and it does not corrode.
Items made out of bronze will tarnish even more quickly than sterling silver, but the advantage is that it can easily have its shine restored. It is recommended that you prevent bronze from getting exposed to moisture because moisture accelerates the tarnishing of metal.